When we talk about managing bacterial infections, antibiotics are our frontline heroes. Among the most versatile and widely used are cephalosporins, a powerhouse class derived from a humble mold. These broad-spectrum beta-lactams have evolved over decades, categorized into five distinct generations, each with its own battle plan against specific bacterial foes. Today, we're diving into the unique world of Overview & Mechanism of Action of 2nd Gen Cephalosporins – understanding how these crucial drugs work, what they target, and where they fit into modern medicine.
Think of cephalosporins as specialized tools in a doctor’s toolkit. The second-generation models represent a significant upgrade, broadening their reach from their first-generation predecessors, particularly against a wider array of gram-negative bacteria, while still retaining some gram-positive punch. They're not just a minor tweak; they’re a strategic development designed for more complex skirmishes within the body.
At a Glance: 2nd Gen Cephalosporins
- What They Are: Broad-spectrum beta-lactam antibiotics.
- Mechanism: Inhibit bacterial cell wall synthesis by binding to penicillin-binding proteins (PBPs).
- Key Strength: Improved coverage against certain gram-negative bacteria compared to 1st gen.
- Notable Members: Cefuroxime (good for H. influenzae), Cephamycins (Cefoxitin, Cefotetan – excellent anaerobic and Bacteroides coverage).
- Common Uses: Respiratory infections, UTIs, skin/soft tissue, bone/joint, gynecological, and intra-abdominal infections.
- Crucial Considerations: Generally require renal dose adjustment, don't cover Enterococci, Listeria, or most MRSA.
Understanding Cephalosporins: A Quick Primer
Before we zoom in on the second generation, let's establish the foundational principles of cephalosporins as a whole. Discovered from the mold Acremonium (formerly Cephalosporium acremonium), these antibiotics share a common core: a beta-lactam ring structure. This ring is their molecular "key" to disrupting bacterial life.
Their classification into generations isn't arbitrary. It reflects an ongoing evolution in their antimicrobial spectrum (which bacteria they can kill) and pharmacokinetic properties (how the body handles them). Each generation typically builds upon the last, often expanding coverage against more resistant strains or targeting new types of infections. From a clinical perspective, they are indispensable, frequently chosen for their wide efficacy and generally favorable safety profile. They're a testament to continuous innovation in the fight against infectious diseases.
The Blueprint of Battle: How Cephalosporins Work
To truly appreciate second-generation cephalosporins, we must first grasp their fundamental mechanism of action. This isn't just academic; understanding how they work clarifies why they are effective against certain bacteria and not others.
The secret lies in the bacterial cell wall – a crucial protective barrier that maintains the cell's shape and integrity, preventing it from bursting due to osmotic pressure. Imagine a brick wall surrounding a house; if that wall is compromised, the house becomes vulnerable.
Cephalosporins, like other beta-lactam antibiotics, are master saboteurs of this bacterial "brick wall." Here’s the step-by-step breakdown:
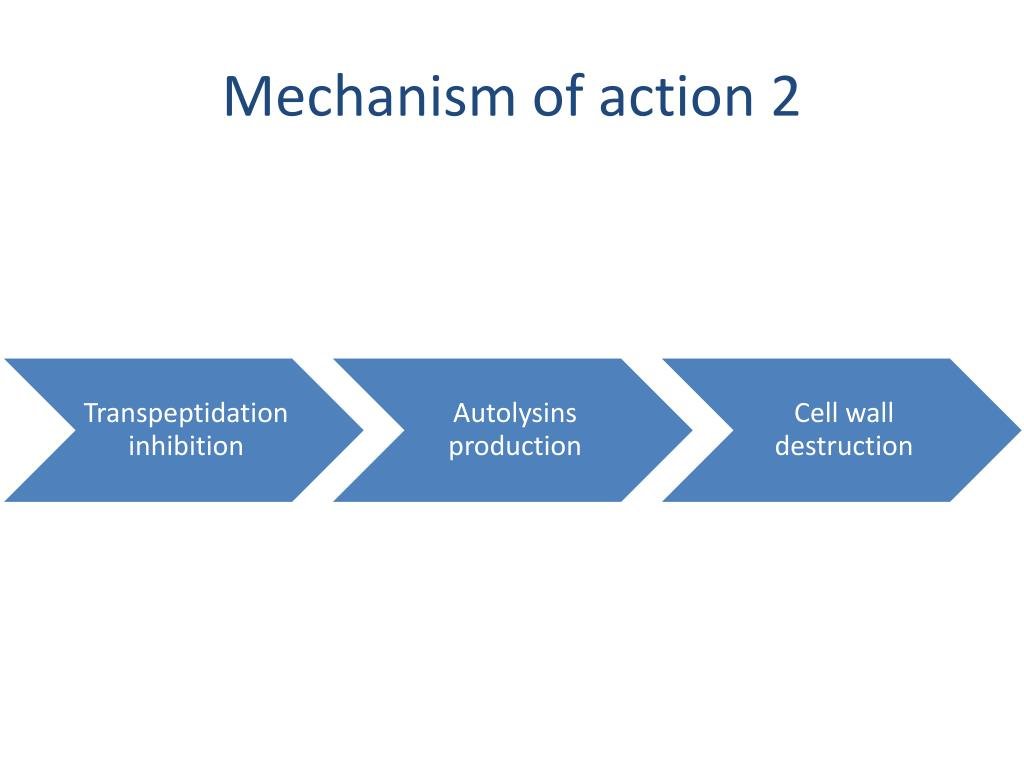
- The Target: Penicillin-Binding Proteins (PBPs). Bacteria construct their cell walls using enzymes called Penicillin-Binding Proteins (PBPs). These PBPs are critical for the final stages of peptidoglycan synthesis, the complex polymer that forms the backbone of the cell wall. Specifically, PBPs facilitate a process called transpeptidation, which cross-links peptidoglycan strands, making the wall strong and rigid.
- The Attack: Beta-Lactam Ring Binding. Cephalosporins possess a distinctive beta-lactam ring in their chemical structure. This ring is structurally similar to the D-alanyl-D-alanine portion of the peptidoglycan precursor that PBPs normally bind to. Because of this mimicry, the beta-lactam ring acts as a decoy, irreversibly binding to the active site of the PBPs.
- The Blockade: Inhibiting Transpeptidation. Once a cephalosporin binds to a PBP, it effectively "handcuffs" the enzyme, preventing it from performing its crucial transpeptidation function. The cross-linking of peptidoglycan chains stops.
- The Consequence: Compromised Cell Wall Integrity. Without proper cross-linking, the peptidoglycan wall becomes weak, fragile, and structurally unsound. Think of a brick wall where the mortar hasn't set – it just crumbles.
- The Result: Osmotic Lysis and Bactericidal Death. The weakened bacterial cell wall can no longer withstand the high internal osmotic pressure. Water rushes into the cell, causing it to swell and ultimately rupture. This catastrophic failure of the cell wall leads to the death of the bacterial cell. This process, known as osmotic lysis, is what makes cephalosporins bactericidal – they actively kill bacteria, rather than just inhibiting their growth.
This elegant yet devastating mechanism is why cephalosporins are so effective. By targeting a process essential and unique to bacterial survival (they don't have human equivalents for these cell wall components), they can eliminate infections with relatively low toxicity to human cells.
Second-Generation Cephalosporins: Stepping Up the Fight
If first-generation cephalosporins were adept at handling common gram-positive bacteria and a handful of gram-negatives, the second generation represents a strategic evolution. They were designed to bridge a gap, offering a more balanced approach against a broader spectrum of pathogens.
The Evolutionary Leap
Second-generation cephalosporins mark a significant shift in the antimicrobial landscape. While they generally maintain activity against some gram-positive organisms (though often less potent than their first-generation cousins), their real advancement lies in their increased effectiveness against key gram-negative bacteria. This expanded coverage includes organisms like Haemophilus influenzae, Neisseria gonorrhoeae, and some Klebsiella pneumoniae.
However, this enhanced gram-negative punch often comes with a slight trade-off in gram-positive potency. They aren't typically the first choice for serious Staphylococcus aureus infections unless specifically indicated or if resistance patterns necessitate. They find their niche in treating infections where both gram-positive and gram-negative coverage is needed, particularly those involving respiratory or abdominal pathogens.
Key Players and Their Unique Strengths
The second-generation isn't a monolithic group. It includes several important drugs, each with its own nuances and particular strengths:
- Cefuroxime (Ceftin, Zinacef): This is a prominent member, often used for respiratory tract infections. Cefuroxime is especially valued for its increased coverage against Haemophilus influenzae, a common culprit in otitis media (ear infections), sinusitis, and bronchitis. It's available in both oral and intravenous forms, offering flexibility in treatment.
- Cephamycins (Cefoxitin, Cefotetan): These are a special subclass within the second generation, distinguished by an additional methoxy group that makes them more resistant to certain beta-lactamases produced by anaerobic bacteria. Their defining characteristic is their significantly enhanced coverage against anaerobic bacteria, especially Bacteroides fragilis species. This makes them invaluable for infections originating in the gut.
- Cefoxitin: A workhorse for surgical prophylaxis, particularly in abdominal and gynecological surgeries, due to its excellent anaerobic coverage.
- Cefotetan: Similar to cefoxitin but with a longer half-life, allowing for less frequent dosing. It also has a unique side chain that can sometimes interfere with vitamin K metabolism, requiring caution in certain patients.
- Cefaclor (Ceclor (DSC), Raniclor (DSC)): An oral second-generation cephalosporin, sometimes used for respiratory and urinary tract infections.
- Cefprozil (Cefzil): Another oral option, often chosen for upper and lower respiratory tract infections, as well as skin and soft tissue infections.
Each of these agents, while sharing the core second-generation characteristics, brings specific advantages to the clinical table, allowing for tailored antibiotic therapy based on the suspected or confirmed pathogens. Explore 2nd generation cephalosporins to delve deeper into the nuances of each drug.
The Battleground: What 2nd Gens Treat
Given their expanded spectrum, second-generation cephalosporins are crucial in treating a variety of infections. Their ability to tackle both gram-positive and gram-negative pathogens, with a special nod to anaerobes for cephamycins, makes them highly adaptable.
Here’s a look at common scenarios where these antibiotics shine:
- Lower Respiratory Tract Infections (LRTIs): Conditions like community-acquired pneumonia, acute exacerbations of chronic bronchitis, and other chest infections often involve organisms like H. influenzae and K. pneumoniae, for which 2nd gens (especially cefuroxime) provide excellent coverage.
- Upper Respiratory Tract Infections (URTIs): While many URTIs are viral, bacterial sinusitis and otitis media (ear infections) can be effectively treated with drugs like cefuroxime.
- Skin and Soft Tissue Infections: While often associated with gram-positives, mixed infections or those involving specific gram-negative organisms can benefit from second-generation therapy.
- Bone and Joint Infections: These can be challenging, but second-generation cephalosporins may be used in certain contexts, particularly when a broader spectrum is needed.
- Urinary Tract Infections (Serious): For more complicated UTIs or those that haven't responded to narrower spectrum agents, 2nd gens can be a suitable choice.
- Gynecological Infections: Cephamycins (cefoxitin, cefotetan) are particularly useful here due to their strong anaerobic coverage, addressing polymicrobial infections common in the pelvic area.
- Intra-abdominal Infections: This is a prime area for cephamycins. Infections originating in the gut, such as diverticulitis, appendicitis, or peritonitis, frequently involve a complex mix of aerobic and anaerobic bacteria (Bacteroides species being a key anaerobic pathogen). Cefoxitin and Cefotetan are often chosen as empirical therapy or for surgical prophylaxis in these settings.
- Blood Infections (Sepsis): When initial empiric broad-spectrum coverage is required for suspected bacterial sepsis, particularly in cases where the source might involve mixed aerobic/anaerobic flora, 2nd gens can play a role, often in combination with other agents.
- Meningitis in Children: Cefuroxime has historically been used for bacterial meningitis in children, particularly caused by H. influenzae, though often superseded by third-generation options for broader CNS penetration and coverage today.
The key takeaway is that their utility often comes down to the suspected pathogens and the specific site of infection. For instance, the anaerobic coverage of cephamycins makes them almost irreplaceable for certain intra-abdominal infections.
Navigating the Side Effects: What to Expect
While second-generation cephalosporins are generally well-tolerated, like all medications, they come with potential side effects. Most of these are mild, transient, and resolve once the medication is stopped. However, patients and clinicians should be aware of both common and rare, but more serious, adverse reactions.
Common (Usually Mild and Transient)
These side effects often appear early in the course of treatment and typically subside within a day or two:
- Gastrointestinal Distress: This is perhaps the most frequent complaint, including:
- Stomach discomfort or pain
- Nausea and vomiting
- Decreased appetite
- Diarrhea (can range from mild to, rarely, Clostridioides difficile-associated diarrhea)
- Neurological:
- Dizziness (especially with rapid IV infusion)
- Dermatological:
- Skin rash (often mild and non-allergic)
- Other:
- Difficulty in breathing (mild, not severe allergic reaction)
- Low blood pressure (especially with rapid IV infusion)
- Thrombocytopenia (decreased platelet count, usually mild and reversible)
- Fever (drug-induced)
- Yeast infections (oral thrush, vaginal candidiasis, due to disruption of normal flora)
- Increased liver enzymes (transaminases), usually asymptomatic and reversible.
Rare but Serious Side Effects
These warrant immediate medical attention:
- Hypersensitivity Reactions: This is the most critical concern. Ranging from severe skin rashes (e.g., Stevens-Johnson syndrome) to anaphylaxis (a life-threatening allergic reaction with difficulty breathing, swelling, and a sudden drop in blood pressure). Patients with a history of penicillin allergy should be carefully assessed due to potential cross-reactivity, though it's generally lower for cephalosporins than previously thought (around 2-5%).
- Drug-Induced Immune Hemolytic Disease (DIIHD): A rare but serious condition where the antibiotic triggers the immune system to attack and destroy the body's own red blood cells. Symptoms can include fatigue, pallor, dark urine, and jaundice.
- Nephrotoxicity: While less common than with some other antibiotic classes, renal impairment can occur, especially in patients with pre-existing kidney disease or when combined with other nephrotoxic drugs. Monitoring renal function is important.
- Seizures: In very high doses or in patients with impaired renal function where the drug accumulates, CNS toxicity including seizures can occur.
Patients should always report any unusual or severe symptoms to their healthcare provider. Monitoring for allergic reactions is paramount, particularly during the first few doses.
Knowing Their Limits: Where 2nd Gens Don't Go
Despite their broad utility, it's crucial to understand the limitations of second-generation cephalosporins. No single antibiotic is a magic bullet, and inappropriate use can lead to treatment failure or contribute to antibiotic resistance.
The Uncovered Targets
There are specific pathogens or types of infections that second-generation cephalosporins generally do not cover:
- Enterococci: This group of gram-positive bacteria (e.g., Enterococcus faecalis, Enterococcus faecium) is intrinsically resistant to all cephalosporins.
- Listeria: Listeria monocytogenes, another gram-positive bacterium, is also not covered by cephalosporins.
- MRSA (Methicillin-Resistant Staphylococcus aureus): With very few exceptions (like the fifth-generation cephalosporin ceftaroline), cephalosporins are ineffective against MRSA. Relying on a 2nd gen cephalosporin for suspected MRSA would be a significant treatment error.
- Atypicals: Bacteria like Mycoplasma pneumoniae, Chlamydia pneumoniae, and Legionella pneumophila (often causing "atypical pneumonia") lack a traditional peptidoglycan cell wall, rendering beta-lactams like cephalosporins ineffective.
- Pseudomonas aeruginosa: While some later-generation cephalosporins (like ceftazidime from 3rd gen or cefepime from 4th gen) are excellent for Pseudomonas, second-generation agents generally lack reliable activity against this notoriously resistant gram-negative pathogen.
- Extended-Spectrum Beta-Lactamase (ESBL) producing bacteria: Many 2nd gen cephalosporins are susceptible to hydrolysis by ESBL enzymes, meaning they would be ineffective against bacteria producing these enzymes, even if the bacteria are theoretically in their spectrum.
Important Considerations for Use
Effective and safe antibiotic therapy isn't just about choosing the right drug; it's also about managing patient factors and potential interactions:
- Allergies: Always inquire about drug allergies, especially to penicillins and other cephalosporins. While the cross-reactivity rate is low, it's a critical safety measure.
- Renal Dose Adjustment: Most cephalosporins, including the second generation, are primarily eliminated by the kidneys. Therefore, dose adjustments are necessary for patients with renal dysfunction to prevent drug accumulation and potential toxicity. Close monitoring of kidney function (e.g., creatinine clearance) is essential. A notable exception among cephalosporins is ceftriaxone (a third-generation drug), which has dual renal and hepatic elimination and typically does not require renal adjustment unless severe hepatic and renal impairment are present.
- Specific Drug Choices for Specific Scenarios:
- For serious Pseudomonas infections, you'd bypass 2nd gens entirely and opt for 3rd generation (ceftazidime) or 4th generation (cefepime) cephalosporins, or other antipseudomonal agents.
- If MRSA is a strong consideration, ceftaroline (a 5th gen cephalosporin) or other non-cephalosporin drugs like vancomycin or linezolid would be appropriate.
- For excellent central nervous system (CNS) and tissue penetration (e.g., for meningitis), ceftriaxone often stands out due to its superior pharmacokinetic profile and once-daily dosing.
Understanding these limitations and considerations is paramount for clinicians to make informed decisions, ensuring patients receive the most appropriate and effective treatment while minimizing risks.
Smart Prescribing: Key Takeaways for Clinicians & Patients
The journey through the Overview & Mechanism of Action of 2nd Gen Cephalosporins reveals a sophisticated class of antibiotics with distinct advantages and important limitations. For both healthcare providers and patients, smart prescribing and responsible use are paramount.
For Clinicians:
- Know Your Spectrum: Recognize that 2nd gen cephalosporins offer a valuable balance of gram-positive and enhanced gram-negative coverage, with cephamycins providing critical anaerobic activity. This makes them ideal for specific mixed infections, especially respiratory, skin, and intra-abdominal.
- Target Appropriately: Avoid using 2nd gens where they lack activity (e.g., against Enterococci, Listeria, MRSA, Pseudomonas, or atypicals). Choosing the wrong antibiotic not only fails to treat the infection but also contributes to antibiotic resistance.
- Mind the Renals: Always consider renal function and adjust dosages accordingly for most 2nd gen cephalosporins.
- Prophylaxis Power: Remember cephamycins like cefoxitin for surgical prophylaxis, particularly in procedures where anaerobic bacteria are a concern.
- Stay Updated: Antibiotic resistance patterns evolve. Regularly review local antibiograms to ensure your empiric choices remain effective.
For Patients:
- Communicate Clearly: Always inform your doctor about all your allergies, especially to other antibiotics.
- Complete the Full Course: Even if you feel better, finish the entire prescribed course of antibiotics. Stopping early can lead to recurring infections and promote antibiotic resistance.
- Report Side Effects: Pay attention to any unusual symptoms, particularly severe skin rashes, difficulty breathing, or persistent severe diarrhea, and report them immediately to your healthcare provider.
- Understand Why: Don't hesitate to ask your doctor or pharmacist questions about why a particular antibiotic was chosen for your infection. Understanding the rationale empowers you to be a more active participant in your care.
Second-generation cephalosporins are powerful tools in our arsenal against bacterial infections. Their mechanism of action, by disrupting bacterial cell wall synthesis, is a testament to targeted therapy. By understanding their strengths, nuances, and limitations, we can harness their full potential, ensuring effective treatment while preserving their efficacy for generations to come.